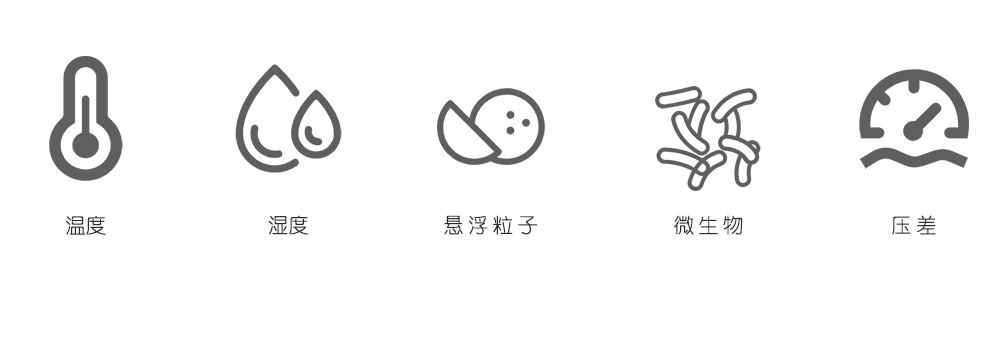
As an important part of pharmaceutical production quality control, the HVAC system in pharmaceutical manufacturing primarily participates in control and monitoring of air temperature, humidity, airborne particles, and microorganisms in the production environment to ensure that environmental parameters meet drug quality requirements, prevent air pollution and cross-contamination, and provide a comfortable environment for operators. Additionally, the pharmaceutical HVAC system can also reduce and prevent adverse effects of drugs on personnel during production and protect the surrounding environment.
The Good Manufacturing Practices for Pharmaceutical Products (2010 Revision), as the primary design basis for cleanroom construction in the pharmaceutical industry, put forward clear requirements for the air cleanliness levels in key pharmaceutical production processes. When pharmaceutical production processes or products have special temperature and humidity requirements, these parameters should be determined based on the specific process and product requirements. When pharmaceutical production has no special temperature and humidity requirements, Grade A, B, and C cleanrooms should maintain a temperature of 20°C-24°C with 45%-60% relative humidity; Grade D cleanrooms should maintain a temperature of 18°C-26°C with 45%-65% relative humidity; and the personnel purification and living areas should maintain a temperature of 16°C-20°C in winter and 26°C-30°C in summer.
The "Good Manufacturing Practice for Pharmaceutical Products" (2010 Revision) clearly defines the requirements for air cleanliness and microbial particle concentration limits in pharmaceutical production environments, with cleanrooms classified into four grades: A, B, C, and D.
|
Cleanliness Grade |
Maximum Permitted Particles per Cubic Meter |
|||
|
At Rest |
In Operation |
|||
|
≥0.5μm |
≥5.0μm |
≥0.5μm |
≥5.0μm |
|
|
Grade A |
3520 |
20 |
3520 |
20 |
|
Grade B |
3520 |
29 |
352000 |
2900 |
|
Grade C |
352000 |
2900 |
3520000 |
29000 |
|
Grade D |
3520000 |
29000 |
No requirement |
No requirement |
|
Cleanliness Grade |
Airborne viable particles
cfu/mm³ |
Settle plates (ø90mm)
cfu/4 hours |
Surface microorganisms |
|
|
Contact plates
cfu/plate |
5-finger glove
cfu/glove |
|||
|
Grade A |
<1 |
<1 |
<1 |
<1 |
|
Grade B |
10 |
5 |
5 |
5 |
|
Grade C |
100 |
50 |
25 |
- |
|
Grade D |
200 |
100 |
50 |
- |
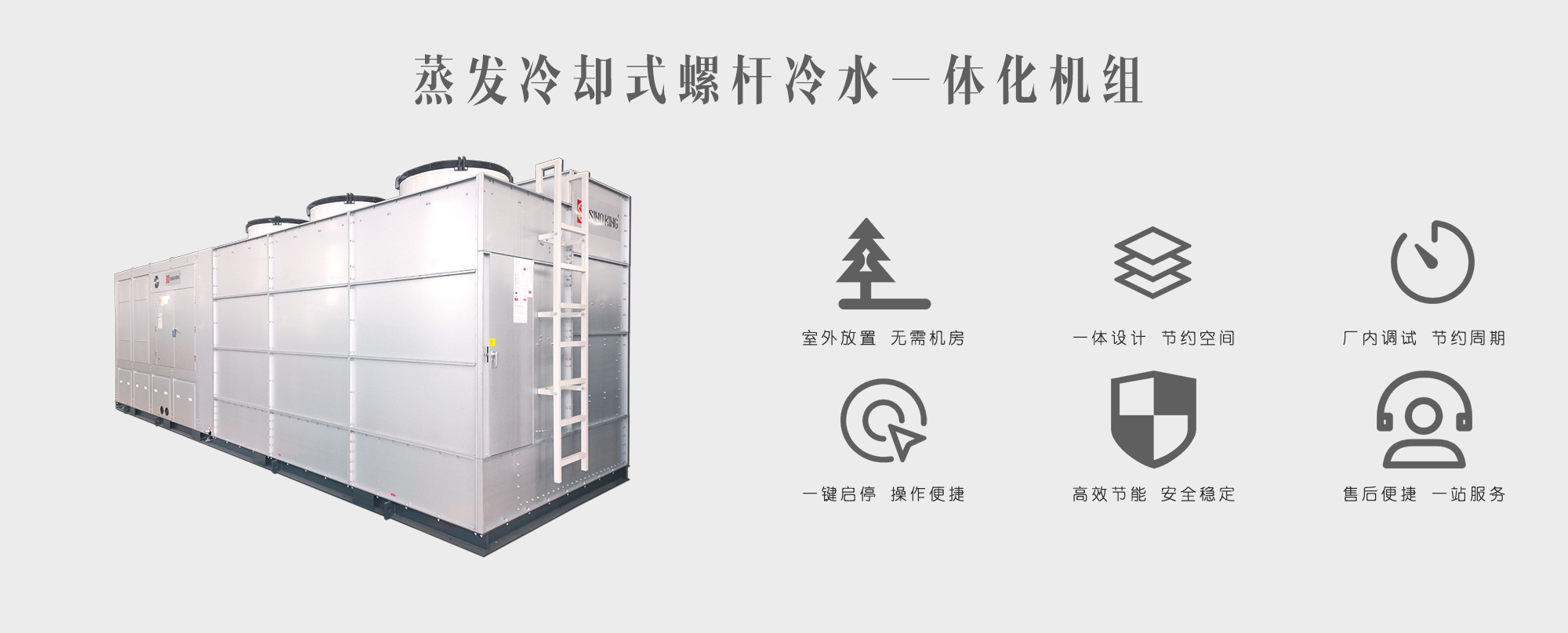
To address pharmaceutical factories' requirements for large cooling capacity and high-frequency usage of air conditioning systems, SINO KING recommends a flooded screw chiller unit with large cooling capacity for facilities with dedicated cold source rooms, and an evaporative cooling integrated chiller unit for facilities without separate cold source rooms. The air handling side utilizes purification-type packaged air conditioning units, with fresh air purification-type DC variable frequency air-cooled direct-expansion packaged air conditioners specifically recommended for anode control laboratories. SINO KING's custom-designed evaporative cooling integrated chiller, a substitute for traditional chiller equipment, improves the utility value of factory buildings while delivering high energy efficiency ratios and reducing electricity costs. The purification-type packaged air conditioning units can be configured with various sections - fresh air section, primary filter, preheating, return air, medium efficiency filter, sub-HEPA filter, HEPA filter, surface air cooler, eliminator, heating, humidification, airflow stabilization, heat recovery, dehumidification, sterilization, sound attenuation, supply fans, and air discharge – according to different purification application scenarios to meet temperature, humidity, and cleanliness requirements for different pharmaceuticals and different production processes.